India approves world’s 1st intra-nasal Covid vaccine
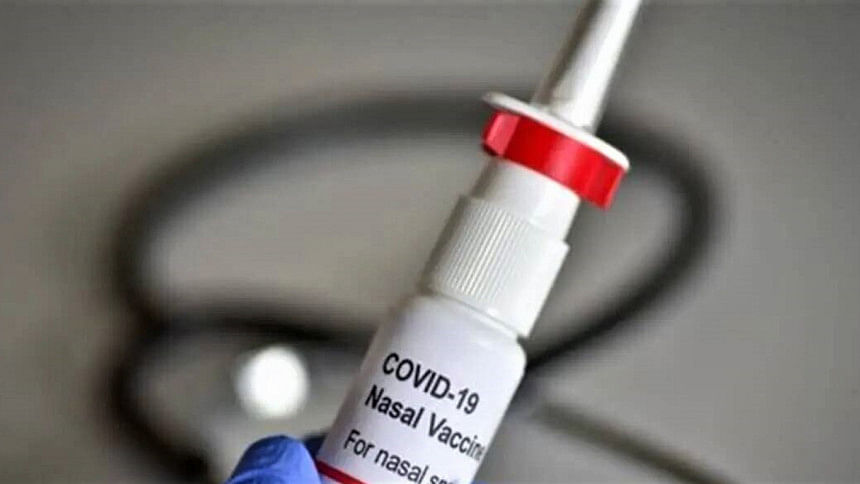
The world's first intra-nasal Covid vaccine developed by India has got the approval from the country's Central Drugs Standard Control Organisation for restricted use.
The vaccine will be used as booster doses for people aged 18 and above only in emergency situations.
The vaccine has been developed by Bharat Biotech International Limited (BBIL) which had also manufactured India's first indigenous Covid vaccine, reports our New Delhi correspondent.
The intra-nasal vaccine was evaluated in Phases I, II and III clinical trials with successful results, the Indian health ministry said in a statement yesterday, adding the phase-III trials of the intra-nasal vaccine were conducted on 3,100 people for safety and immunogenicity.
The nasal delivery system has been designed and developed to be cost-effective in low- and middle-income countries. It is stable at 2-8°C temperature for easy storage and distribution, the ministry statement said.
Heterologous booster dose studies were conducted for safety and immunogenicity on 875 people where a booster dose (3rd dose) of BBV154 intranasal vaccine was administered to study participants who were previously vaccinated with Covid vaccines.
The intra-nasal vaccine has the double benefit of enabling faster development of Covid variant-specific vaccines and easy nasal delivery that enables mass immunisation to protect from emerging variants of concern. It promises to become an important tool in mass vaccinations during pandemics and endemics.

 For all latest news, follow The Daily Star's Google News channel.
For all latest news, follow The Daily Star's Google News channel. 


Comments