The Race for the First Vaccine
Since the advent of vaccines by Edward Jenner in 1798, the course of medical history has changed forever. Vaccines are one of the most successful health interventions, saving three million lives every year.
As we close in on December, almost a year after the first Covid-19 case sprung up, the numbers of people infected have anything but declined. Much to the dismay of anti-vaxxers, there have been vast advancements towards several immunization biologics. China, Russia, the United Arab Emirates and the United Kingdom are already availing some of the options; there are seven vaccines approved for early or limited use.
The race for a vaccine has led to a variety of projects based on mRNA, viral vectors, proteins and inactivated forms of SARS-CoV-2. Some are also being repurposed -- existing vaccines being redesigned to combat the coronavirus.
There are at least 87 pre-clinical vaccines and 58 clinical vaccines being tested and no approved vaccine yet for full use. Whoever reaches the finish line first, the vaccine should not be overvalued in terms of its efficiency and market dominance. More vaccines are to emerge over time and are predicted to outdo one another. Meanwhile anyone remotely being capable of fighting the virus would be heralded as the ultimate saviour.
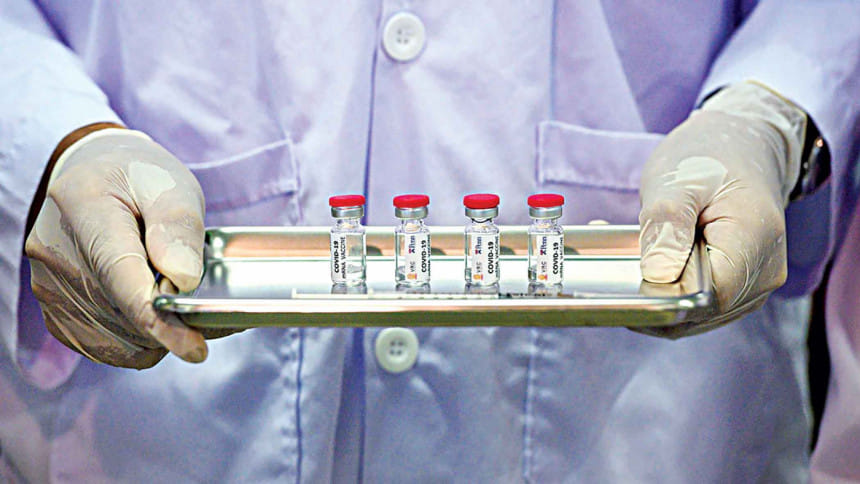
Recently, three vaccines at Phase III trials have been taking the headlines by storm with staggering efficiency data. We know what these are. And each comes with certain logistical challenges to beat when it comes to mass production and consequently, distribution.
Pfizer and BioNTech's BNT162b2 and Moderna's mRNA-1273 are the same in a sense that both are made of a genetic molecule, mRNA which requires extreme cold conditions to keep it intact and functional. While Moderna requires a significantly lower temperature of -20 °C, Pfizer demands that it be -70 °C. However, both imperatives are posing outstanding difficulties in transport and storage in hospitals and clinics, from bottles to needles. Like many other vaccines, these too require two doses approximately a month apart, increasing the delivery load. It is said that the access and distribution is now a much greater problem than creating the vaccine itself. As countries place orders for millions of doses, these vaccines may be manufactured in one continent and required to be shipped to another, all the while maintaining arctic icy conditions.
This means that the planes, trucks, trailers, warehouses, and the provider facilities will have to be equipped with ultra-cold freezers for the first time. For developing and underdeveloped nations, where a regular fridge is a luxury, experts have expressed concerns and urged to find solutions for efficient delivery. The problem is that the countries that need vaccines the most, are the least ready to receive them.
The sub-zero temperature naturally calls for more dry ice that has been in shortage in the wake of the pandemic. And, cold-resistant glass vials because glass cracks under extreme cold. To tackle this, Corning is on track to produce millions of a new type of pharmaceutical-grade glass vials to contain the frozen vaccine.
Pfizer has developed a special kind of GPS-tracked "cool box" meant to store 1,000-5,000 vaccines for up to 15 days, if the dry ice stock is carefully replenished. The boxes should be closed within a minute of opening and to add more, should not be opened more than twice in a day. When diluted, each vial makes up for 5 doses. Once thawed, the undiluted vial is usable for only 5 days while a diluted one lasts for 6 hours in a refrigerator. The strict terms imply that providers need to accurately predict how many arms to inject on a daily basis. Otherwise there is either a waste or a deficit.
From manufacturing to distribution to provider facility to administration, the cold-chain needs to stay put. Logistics companies worldwide are striving to upgrade their cold infrastructure. The problem has arisen because these vaccines have been tested to be at least 90 percent effective in said conditions and have not had the chance to be investigated differently owing to the urgency of the matter.
The other leading vaccine by AstraZeneca and Oxford University, AZD1222, can be served chilled at 2 to 8 °C meaning no necessity of deep-freezers. It lasts as long as six months however, its efficacy ranges from 62 to 90 percent. Requiring two doses, it is priced at around USD 3 to 4 per dose, much cheaper than Pfizer's (USD 19.50) and Moderna's (USD 32 to 37). This vaccine is very feasible, especially for rural areas, to use with existing healthcare settings. But AstraZeneca and Oxford have faced criticism for lack of transparency with the results from trials. And most recently, a trial volunteer has claimed to suffer severe side-effects from the vaccine. However, the manufacturer, Serum Institute of India (who have also been conducting trials in India as per the manufacturing agreement), has denied the allegations and has assured that the product will be released only when proven to be completely safe.
There are still certain questions to be answered. How long does immunity last? So far, it is unknown if the mRNA vaccines affect infections. Only the clinical symptoms of SARS-CoV-2 were successfully combated. Preventing infections reduces the spread while preventing symptoms decreases the rate of hospitalizations. So would it stop asymptomatic transmission? We don't know, but the future is not as dark as we once thought.
References
1. The New York Times (December 2, 2020). Coronavirus Vaccine Tracker.
2. The New York Times (September 18, 2020). How to Ship a Vaccine at –80°C, and Other Obstacles in the Covid Fight.
3. Business Insider (November 13, 2020). Pfizer's vaccine relies on a 'cold chain' that keeps the shots colder than a freezer. Here's how it works.
4. The Washington Post (November 30, 2020). What you need to know about the Pfizer, Moderna and AstraZeneca vaccines.
5. Healthline (November 29, 2020). How much will it cost to get a COVID-19 vaccine?

 For all latest news, follow The Daily Star's Google News channel.
For all latest news, follow The Daily Star's Google News channel. 



Comments