Antigen test still a far cry
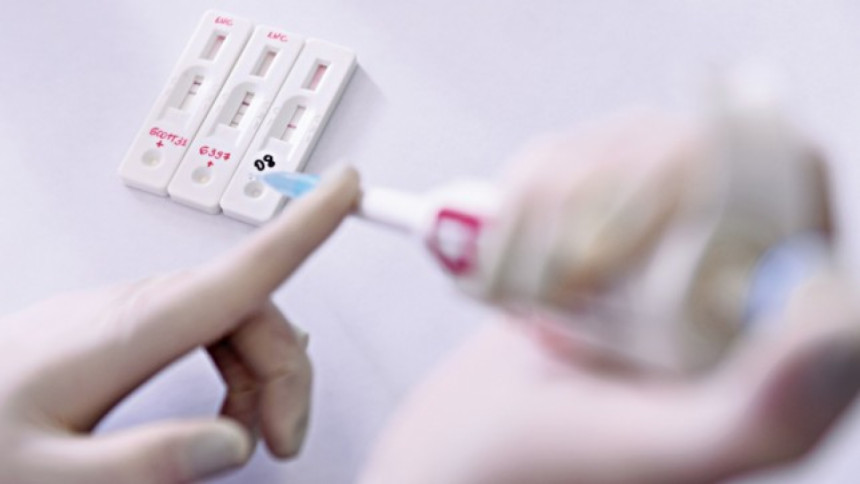
The government has not yet been able to launch the antigen-based rapid testing kit at the designated healthcare facilities even two months after approval.
Officials at the health directorate said the procurement of the kits has not been completed yet, thus causing the delay.
The kit was approved in order to ensure a greater number of tests and quicker results.
"The procurement of the kits from the Central Medical Store Depot is underway. We will start the antigen-based rapid testing as soon as we receive them," Md Habibur Rahman, spokesperson for the DGHS told The Daily Star.
Sources said the Directorate General of Drug Administration (DGDA) has not received no objection certificates (NOC) from any organisation about importing the kits.
The NOC from the DGDA is mandatory for importing any medical device into the country.
Until now, Bangladesh relied on the RT-PCR tests -- considered the gold standard for Covid-19 detection -- since the pandemic hit the country in March. But the process of testing is expensive and time consuming.
Following recommendations given by the National Technical Advisory Committee, the health services division of the health ministry on September 17 approved the antigen rapid testing kit after months of bureaucratic exercises.
It aimed at setting up antigen testing facilities at 39 public hospitals and specialised institutes in areas which did not have RT-PCR testing facilities.
The country is conducting around 15,000 tests a day in 117 authorised laboratories. It is ranked at the bottom in the world in terms of conducting Covid-19 tests considering its population of over 160 million.
The decision to launch antigen testing was taken primarily to increase the testing capacity at district hospitals where RT-PCR facilities are not available and also at specialised hospitals to treat critical patients.
An antigen test reveals if a person is currently infected with a pathogen and is conducted by taking a saliva sample. It can usually determine within 15 minutes whether a patient is acutely infected and contagious.
A number of countries across the world, including the USA, members of the European Union and India, are conducting antigen tests to contain the second wave.
Although the test is less accurate than a complete PCR analysis, the major advantages are quick results and the possibility to use it directly on site, according to experts.
The DGDA has set 90 percent sensitivity and 95 percent specificity as the standard for the approval of any antigen kit.
But there are only two or three organisations in the world which have been able to fulfil the standard set by the DGDA, according to the officials.
Meanwhile, the Institute of Epidemiology, Disease Control and Research (IEDCR) has been running feasibility tests of the antigen kits from at least three foreign companies for nearly two months.
One kit has shown more than 80 percent sensitivity, according to an IEDCR official.
Currently, the IEDCR is running trials of the second kit, said the official preferring anonymity.
Speaking to The Daily Star on Wednesday, IEDCR Director Prof Tahmina Shirin said, "It doesn't matter what is the result of our trial is. Any kits which has the FDA's approval can be imported as per the DGDA standard."


 For all latest news, follow The Daily Star's Google News channel.
For all latest news, follow The Daily Star's Google News channel. 



Comments