Apple receives FDA approval to turn AirPods Pro into hearing aids
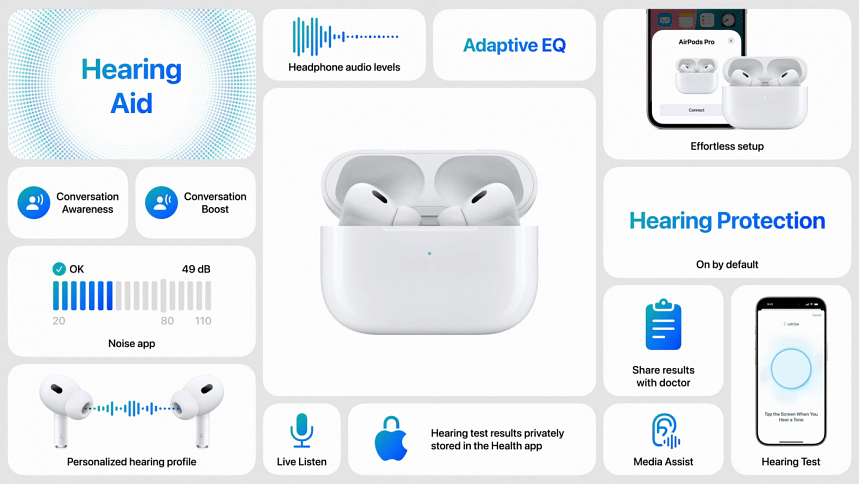
Apple has received authorization from the U.S. Food and Drug Administration (FDA) to transform its second-generation AirPods Pro into over-the-counter (OTC) hearing aids. This marks a significant step in Apple's commitment to hearing health, making the AirPods Pro the first OTC hearing aid software device approved by the FDA. The feature is expected to be available later this fall.
The FDA approval follows a clinical study that involved 118 individuals with perceived mild to moderate hearing loss. The study found that users who self-fitted the hearing aid functionality in the AirPods Pro experienced similar benefits to those who had professional fittings. The new hearing aid feature is one of several enhancements Apple plans to introduce to its second-generation AirPods Pro.
In addition to the hearing aid capability, the AirPods Pro will feature a Hearing Protection mode, designed to safeguard users' ears in loud environments such as concerts. This mode will be enabled by default and aims to preserve the natural sound quality in noisy settings. Apple will also introduce a Hearing Test, allowing users to create personalized audio profiles by responding to tones played through the earbuds, with the results available in the Apple Health app.
This development could make hearing aids more accessible, reducing the stigma and high costs associated with traditional devices. According to the World Health Organization, around 1.5 billion people globally live with some degree of hearing loss, making Apple's new feature potentially impactful on a large scale.
Apple's commitment to hearing health is part of a broader initiative that already includes features on its devices to warn users when they are exposed to dangerously loud environments or audio levels that could cause permanent hearing damage. The FDA's approval was granted under the De Novo classification, which applies to novel, low- to moderate-risk devices without prior legal equivalents.

 For all latest news, follow The Daily Star's Google News channel.
For all latest news, follow The Daily Star's Google News channel. 









Comments